
北大医学——望石智慧联合实验室正式挂牌
9月28日上午,北京大学举行了校企联合研发平台集体签约仪式(北大医学办学 110 周年系列活动),北大医学——望石智慧AI生物医药数据技术协同创新联合实验室正式挂牌。
Just another WordPress site

9月28日上午,北京大学举行了校企联合研发平台集体签约仪式(北大医学办学 110 周年系列活动),北大医学——望石智慧AI生物医药数据技术协同创新联合实验室正式挂牌。
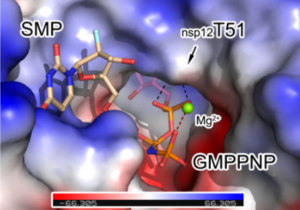
Decoration of cap on viral RNA plays essential roles in SARS-CoV-2 proliferation. Here we report a mechanism for SARS-CoV-2 RNA capping and document structural details at atomic resolution. The NiRAN domain in polymerase catalyzes the covalent link of RNA 5’ end to the first residue of nsp9 (termed as RNAylation), thus being an intermediate to form cap core (GpppA) with GTP catalyzed again by NiRAN. We also reveal that triphosphorylated nucleotide analogue inhibitors can be bonded to nsp9 and fit into a previously unknown ‘Nuc-pocket’ in NiRAN, thus inhibiting nsp9 RNAylation and formation of GpppA. S-loop (residues 50-KTN-52) in NiRAN presents a remarkable conformational shift observed in RTC bound with sofosbuvir monophosphate, reasoning an ‘induce-and-lock’ mechanism to design inhibitors. These findings not only improve the understanding of SARS-CoV-2 RNA capping and the mode of action of NAIs, but also provide a strategy to design antiviral drugs.